Abstract
Background:
The World Health Organization (WHO)-defined criteria for acute myeloid leukemia (AML), including blast phase myeloproliferative neoplasms (MPN-BP), includes the presence of ≥20% blasts in the peripheral blood (PB), regardless of blast content in the bone marrow (BM) (Blood 2016;127:2391) . It is currently unclear if this 20% threshold is valid for primary myelofibrosis (PMF), in terms of distinguishing PB-defined MPN-BP from either chronic phase disease with excess blasts (PMF-EB; defined by the presence of 5-19% circulating blasts) or BM-defined MPN-BP.
Methods:
Diagnoses of MPN, including PMF and MPN-BP, were according to WHO criteria (Blood 2016;127:2391). The study population included chronic phase PMF patients with 5-19% circulating blasts (PMF-EB) and MPN patients with blast phase disease (MPN-BP), defined by the presence of ≥20% blasts in PB only (PB-defined MPN-BP) or BM (BM-defined MPN-BP). Statistical analyses for PMF-EB considered clinical and laboratory data collected at the time of documented PB blast count of ≥5% and for MPN-BP, date of leukemic transformation. Phenotypic and prognostic comparisons considered four distinct categories: i) chronic phase disease with 5-9% circulating blasts (PMF-EB-1), ii) chronic phase disease with 10-19% circulating blasts (PMF-EB-2), iii) MPN-BP defined by ≥20% BM blasts (BM-defined) and iv) MPN-BP defined by ≥20% blasts in PB only (PB-defined).
Results:
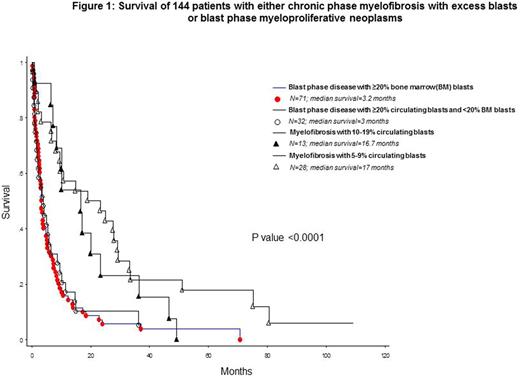
The total number of study patients was144 and included 41 patients with PMF-EB and 103 with MPN-BP; the 41 patients with PMF-EB included 28 with PMF-EB-1 (median age 69 years; 61% males) and 13 with PMF-EB-2 (median age 64 years; 54% males); the 103 patients with MPN-BP included 71 BM-defined (median age 68 years; 69% males) and 32 PB-defined (median age 66 years; 72% males) MPN-BP (p=0.6 for age and 0.6 for gender distribution). The four operational groups were also similar in their need for red cell transfusions (p=0.4), hemoglobin level (p=0.9), leukocyte count (p=0.9) and driver mutational status (p=0.3), including incidence of type 1/like CALR mutations (11% for PMF-EB-1 vs 15% for PMF-EB-2 vs 17% for PB-defined MPN-BP vs 19% for BM-defined MPN-BP). Significant differences were noted for platelet count (lower for both PB and BM defined MPN-BP; p=0.004) and incidence of abnormal karyotype (p=0.02) and unfavorable karyotype (22% for PMF-EB-1, 62% PMF-EB-2, 61% PB-defined MPN-BP and 49% BM-defined MPN-BP; p=0.03). Survival data showed significant difference in favor of PMF-EB, compared to both BM- and PB-defined MPN-BP, while they were similar for PMF-EB-1 vs PMF-EB-2 and for PB- vs BM-defined MPN-BP (Figure 1).
Conclusions:
The observations from the current study support the prognostic validity of current WHO criteria in distinguishing chronic phase PMF with excess blasts (PMF-EB) from PB-defined MPN-BP. The current study also confirms the validity of using circulating blast percentage to define MPN-BP, regardless of BM blast percentage.
No relevant conflicts of interest to declare.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal